Supported by Dr. Osamu Ogasawara and  providing providing  . . |
|
Last data update: 2014.03.03 |
Predict the Hybridization Efficiency of Probe/Target Sequence PairsDescriptionCalculates the Gibbs free energy and hybridization efficiency of probe/target pairs at varying concentrations of the denaturant formamide. Usage
CalculateEfficiencyArray(probe,
target,
FA = 0,
dGini = 1.96,
Po = 10^-2.0021,
m = 0.1731,
temp = 42,
deltaGrules = NULL)
Arguments
DetailsThis function calculates the free energy and hybridization efficiency (HE) for a given formamide concentration ([FA]) using the linear free energy model given by: HE = Po*exp[-(dG_0 + m*FA)/RT]/(1+Po*exp[-(dG_0 + m*FA)/RT]) The If ValueA Author(s)Erik Wright DECIPHER@cae.wisc.edu ReferencesYilmaz LS, Loy A, Wright ES, Wagner M, Noguera DR (2012) Modeling Formamide Denaturation of Probe-Target Hybrids for Improved Microarray Probe Design in Microbial Diagnostics. PLoS ONE 7(8): e43862. doi:10.1371/journal.pone.0043862. See Also
Examples
probes <- c("AAAAACGGGGAGCGGGGGGATACTG", "AAAAACTCAACCCGAGGAGCGGGGG")
targets <- c("CAACCCGGGGAGCGGGGGGATACTG", "TCGGGCTCAACCCGAGGAGCGGGGG")
result <- CalculateEfficiencyArray(probes, targets, FA=0:40)
dG0 <- result[, "dG_0"]
HE0 <- result[, "HybEff_0"]
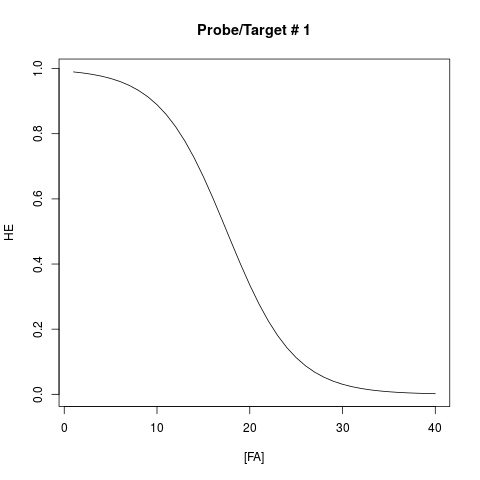
plot(result[1, 1:40], xlab="[FA]", ylab="HE", main="Probe/Target # 1", type="l")
Results
R version 3.3.1 (2016-06-21) -- "Bug in Your Hair"
Copyright (C) 2016 The R Foundation for Statistical Computing
Platform: x86_64-pc-linux-gnu (64-bit)
R is free software and comes with ABSOLUTELY NO WARRANTY.
You are welcome to redistribute it under certain conditions.
Type 'license()' or 'licence()' for distribution details.
R is a collaborative project with many contributors.
Type 'contributors()' for more information and
'citation()' on how to cite R or R packages in publications.
Type 'demo()' for some demos, 'help()' for on-line help, or
'help.start()' for an HTML browser interface to help.
Type 'q()' to quit R.
> library(DECIPHER)
Loading required package: Biostrings
Loading required package: BiocGenerics
Loading required package: parallel
Attaching package: 'BiocGenerics'
The following objects are masked from 'package:parallel':
clusterApply, clusterApplyLB, clusterCall, clusterEvalQ,
clusterExport, clusterMap, parApply, parCapply, parLapply,
parLapplyLB, parRapply, parSapply, parSapplyLB
The following objects are masked from 'package:stats':
IQR, mad, xtabs
The following objects are masked from 'package:base':
Filter, Find, Map, Position, Reduce, anyDuplicated, append,
as.data.frame, cbind, colnames, do.call, duplicated, eval, evalq,
get, grep, grepl, intersect, is.unsorted, lapply, lengths, mapply,
match, mget, order, paste, pmax, pmax.int, pmin, pmin.int, rank,
rbind, rownames, sapply, setdiff, sort, table, tapply, union,
unique, unsplit
Loading required package: S4Vectors
Loading required package: stats4
Attaching package: 'S4Vectors'
The following objects are masked from 'package:base':
colMeans, colSums, expand.grid, rowMeans, rowSums
Loading required package: IRanges
Loading required package: XVector
Loading required package: RSQLite
Loading required package: DBI
> png(filename="/home/ddbj/snapshot/RGM3/R_BC/result/DECIPHER/CalculateEfficiencyArray.Rd_%03d_medium.png", width=480, height=480)
> ### Name: CalculateEfficiencyArray
> ### Title: Predict the Hybridization Efficiency of Probe/Target Sequence
> ### Pairs
> ### Aliases: CalculateEfficiencyArray
>
> ### ** Examples
>
> probes <- c("AAAAACGGGGAGCGGGGGGATACTG", "AAAAACTCAACCCGAGGAGCGGGGG")
> targets <- c("CAACCCGGGGAGCGGGGGGATACTG", "TCGGGCTCAACCCGAGGAGCGGGGG")
> result <- CalculateEfficiencyArray(probes, targets, FA=0:40)
> dG0 <- result[, "dG_0"]
> HE0 <- result[, "HybEff_0"]
> plot(result[1, 1:40], xlab="[FA]", ylab="HE", main="Probe/Target # 1", type="l")
>
>
>
>
>
> dev.off()
null device
1
>
|