Supported by Dr. Osamu Ogasawara and  providing providing  . . |
|
Last data update: 2014.03.03 |
Apply a 'regularized log' transformationDescriptionThis function transforms the count data to the log2 scale in a way
which minimizes differences between samples for rows with small counts,
and which normalizes with respect to library size.
The rlog transformation produces a similar variance stabilizing effect as
Usagerlog(object, blind = TRUE, intercept, betaPriorVar, fitType = "parametric") rlogTransformation(object, blind = TRUE, intercept, betaPriorVar, fitType = "parametric") Arguments
DetailsNote that neither rlog transformation nor the VST are used by the
differential expression estimation in The transformation does not require that one has already estimated size factors and dispersions. The regularization is on the log fold changes of the count for each sample
over an intercept, for each gene. As nearby count values for low counts genes
are almost as likely as the observed count, the rlog shrinkage is greater for low counts.
For high counts, the rlog shrinkage has a much weaker effect.
The fitted dispersions are used rather than the MAP dispersions
(so similar to the The prior variance for the shrinkag of log fold changes is calculated as follows:
a matrix is constructed of the logarithm of the counts plus a pseudocount of 0.5,
the log of the row means is then subtracted, leaving an estimate of
the log fold changes per sample over the fitted value using only an intercept.
The prior variance is then calculated by matching the upper quantiles of the observed
log fold change estimates with an upper quantile of the normal distribution.
A GLM fit is then calculated using this prior. It is also possible to supply the variance of the prior.
See the vignette for an example of the use and a comparison with The transformed values, rlog(K), are equal to
rlog(K_ij) = log2(q_ij) = beta_i0 + beta_ij,
with formula terms defined in The parameters of the rlog transformation from a previous dataset
can be frozen and reapplied to new samples. See the 'Data quality assessment'
section of the vignette for strategies to see if new samples are
sufficiently similar to previous datasets.
The frozen rlog is accomplished by saving the dispersion function,
beta prior variance and the intercept from a previous dataset,
and running Valuea ReferencesReference for regularized logarithm (rlog): Michael I Love, Wolfgang Huber, Simon Anders: Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biology 2014, 15:550. http://dx.doi.org/10.1186/s13059-014-0550-8 See Also
Examples
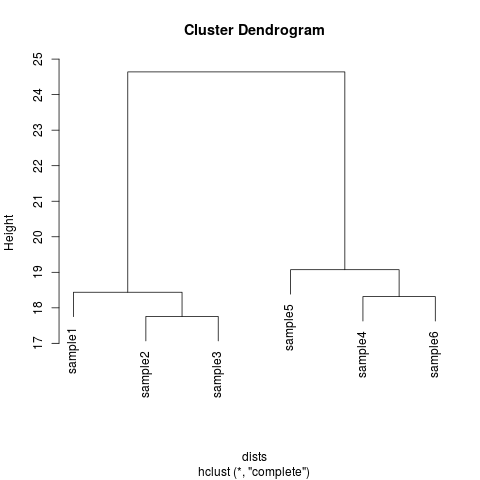
dds <- makeExampleDESeqDataSet(m=6,betaSD=1)
rld <- rlog(dds)
dists <- dist(t(assay(rld)))
plot(hclust(dists))
# run the rlog transformation on one dataset
design(dds) <- ~ 1
dds <- estimateSizeFactors(dds)
dds <- estimateDispersions(dds)
rld <- rlog(dds, blind=FALSE)
# apply the parameters to a new sample
ddsNew <- makeExampleDESeqDataSet(m=1)
mcols(ddsNew)$dispFit <- mcols(dds)$dispFit
betaPriorVar <- attr(rld,"betaPriorVar")
intercept <- mcols(rld)$rlogIntercept
rldNew <- rlog(ddsNew, blind=FALSE,
intercept=intercept,
betaPriorVar=betaPriorVar)
Results
R version 3.3.1 (2016-06-21) -- "Bug in Your Hair"
Copyright (C) 2016 The R Foundation for Statistical Computing
Platform: x86_64-pc-linux-gnu (64-bit)
R is free software and comes with ABSOLUTELY NO WARRANTY.
You are welcome to redistribute it under certain conditions.
Type 'license()' or 'licence()' for distribution details.
R is a collaborative project with many contributors.
Type 'contributors()' for more information and
'citation()' on how to cite R or R packages in publications.
Type 'demo()' for some demos, 'help()' for on-line help, or
'help.start()' for an HTML browser interface to help.
Type 'q()' to quit R.
> library(DESeq2)
Loading required package: S4Vectors
Loading required package: stats4
Loading required package: BiocGenerics
Loading required package: parallel
Attaching package: 'BiocGenerics'
The following objects are masked from 'package:parallel':
clusterApply, clusterApplyLB, clusterCall, clusterEvalQ,
clusterExport, clusterMap, parApply, parCapply, parLapply,
parLapplyLB, parRapply, parSapply, parSapplyLB
The following objects are masked from 'package:stats':
IQR, mad, xtabs
The following objects are masked from 'package:base':
Filter, Find, Map, Position, Reduce, anyDuplicated, append,
as.data.frame, cbind, colnames, do.call, duplicated, eval, evalq,
get, grep, grepl, intersect, is.unsorted, lapply, lengths, mapply,
match, mget, order, paste, pmax, pmax.int, pmin, pmin.int, rank,
rbind, rownames, sapply, setdiff, sort, table, tapply, union,
unique, unsplit
Attaching package: 'S4Vectors'
The following objects are masked from 'package:base':
colMeans, colSums, expand.grid, rowMeans, rowSums
Loading required package: IRanges
Loading required package: GenomicRanges
Loading required package: GenomeInfoDb
Loading required package: SummarizedExperiment
Loading required package: Biobase
Welcome to Bioconductor
Vignettes contain introductory material; view with
'browseVignettes()'. To cite Bioconductor, see
'citation("Biobase")', and for packages 'citation("pkgname")'.
> png(filename="/home/ddbj/snapshot/RGM3/R_BC/result/DESeq2/rlog.Rd_%03d_medium.png", width=480, height=480)
> ### Name: rlog
> ### Title: Apply a 'regularized log' transformation
> ### Aliases: rlog rlogTransformation
>
> ### ** Examples
>
>
> dds <- makeExampleDESeqDataSet(m=6,betaSD=1)
> rld <- rlog(dds)
> dists <- dist(t(assay(rld)))
> plot(hclust(dists))
>
> # run the rlog transformation on one dataset
> design(dds) <- ~ 1
> dds <- estimateSizeFactors(dds)
> dds <- estimateDispersions(dds)
gene-wise dispersion estimates
mean-dispersion relationship
final dispersion estimates
> rld <- rlog(dds, blind=FALSE)
>
> # apply the parameters to a new sample
>
> ddsNew <- makeExampleDESeqDataSet(m=1)
Warning message:
In DESeqDataSet(se, design = design, ignoreRank) :
all genes have equal values for all samples. will not be able to perform differential analysis
> mcols(ddsNew)$dispFit <- mcols(dds)$dispFit
> betaPriorVar <- attr(rld,"betaPriorVar")
> intercept <- mcols(rld)$rlogIntercept
> rldNew <- rlog(ddsNew, blind=FALSE,
+ intercept=intercept,
+ betaPriorVar=betaPriorVar)
>
>
>
>
>
>
>
> dev.off()
null device
1
>
|